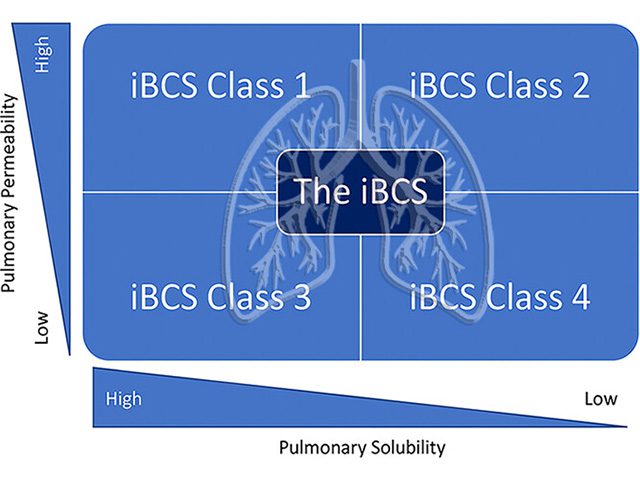
Dr. Per Bäckman, from MVIC member companies Per Backman Consulting and Emmace, is part of the iBSC working group initiated by Product Quality Research Institute (PQRI). They have just published their 3rd paper in a series with one more paper to come. The article series specifies, for the first time, a quantitative inhalation biopharmaceutics classification system (iBCS) for orally inhaled drugs. This is an important and unique effort to classify inhaled drugs and the expectation is that the classification system will be used as a “rule of thumb” by inhalation scientists in the future. Please find the articles no. 1-3 in full via the links below.
iBCS: 1. Principles and Framework of an Inhalation-Based Biopharmaceutics Classification System
iBCS:3. A Biopharmaceutics Classification System for Orally Inhaled Drug Products